PATENT RIGHTS vs. COMPULSORY LICENSING: Examining the challenges and opportunities

Mar. 12, 2025 • Chetna
INTRODUCTION
“The right to swing my fist ends where the other man's nose begins .”
― by Oliver Wendell Holmes Jr.
This quote from Oliver Wendell Holmes Jr. signifies that the private rights of a person end when his/her rights start to harm other people or are restricting the rights of other people. The rights of a person are limited to the point where they start to affect the life and rights of others. The same applies to patent rights. A patent provides a person with the exclusive right of preventing others from selling, using, manufacturing, offering for sale, and importing the invention. Hence, without the permission of the patent holder, no one can sell, offer for sale, manufacture, use, or import the patented invention. However, the concept of compulsory licensing provides an exception to these rights of the patent holder by providing a third party with these rights. Before starting, let us find out what compulsory licensing is.
Key definitions:
Compulsory Licensing:
Compulsory licensing is defined as a permission to use, sell, offer for sale, manufacture, or import against the will or in absence of consent of the patent owner . This allows the third party to conduct these activities even without the consent of the patent holder .
Patent Rights:
Patent rights are defined under Section 48 of the Patents Act of 1970 , as
- In case of product invention: The right to prevent others from selling, using, making, offering for sale, or importing the invention .
- In case of process invention: The right to prevent
- Using of process .
- Using, offering for sale, selling, or importing the product made by that process in India .
The primary use of compulsory licensing is to provide access to affordable drugs and medication to all in their hours of need to save lives . It helps to lower the price of drugs and make them affordable. From the definition above, Patent Rights and Compulsory Licensing are two contrasting concepts. On one hand, the patent provides an exclusive right to the patentee, Compulsory Licensing is an exception to it against the will of the patent holder. It is the remedy to counter the monopoly of the patents . This paper is going to analyze the challenges and opportunities the intersection of these two concepts provides. Why was compulsory licensing needed in the first place, and what impact can it possibly have on innovation and technological advancement?
Research Outline:
This paper will discuss the contrasting nature of patent rights and compulsory licensing. It is divided into four parts, which are illustrated as follows:
Part 1: Concept of Compulsory licensing and associated provision in the Patents Act, 1970 and the Agreement on Trade-Related aspects of intellectual Property Rights (TRIPS Agreement).
Part 2: Instance when the compulsory license was granted and its aftereffects.
Part 3: Comparison of patent rights and compulsory licensing.
Part 4: Author’s point of view and way ahead.
LITERATURE REVIEW
Part 1: Concept of Compulsory licensing and associated provision in the Patents Act, 1970 and the Agreement on Trade-Related aspects of intellectual Property Rights (TRIPS Agreement).
As mentioned above, compulsory licensing is a right provided to a third party against the will of the patent holder to sell, make, offer for sale, use, or import the invention. However, the rights of the patent holder cannot easily be compromised. Certain conditions need to be fulfilled to obtain a compulsory license.
The Patents Act, 1970:
The criteria and requirements to obtain a compulsory license are mentioned under Section 84 of the Patent Act, 1970 . Compulsory Licensing can be granted after three years of the grant of patent by applying to the Controller to get a compulsory license . Compulsory license can be granted if, during those three years, one of the following three grounds occurred:
Hence, one of the three A’s has to be satisfied to obtain a compulsory license.

Figure 1: Three A’s of Compulsory License
Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) :
TRIPS Agreement deals with compulsory licensing for patents under Article 31, “Other Use Without Authorization of the Right Holder ”. Under this article, if there is a condition of national emergency or extreme urgency and,
- Efforts under reasonable terms and conditions made to obtain permission and
- If efforts did not get a positive response within a reasonable time,
then, for non-commercial use, a compulsory license can be granted for the invention to the third party . Provided that,
Hence, compulsory licensing is granted for drugs in dire need when the concerned drug is not available or affordable or accessible in the market to fulfill the consumer demand, and in return for compulsory licensing, the patent holder gets adequate remuneration for the use of his invention. However, the amount of remuneration and terms & conditions for compulsory licensing are not set by the patent holder. It is up to the designated authority authorized to grant compulsory license. In India, the designated authority is the Controller General of Patents, Designs, and Trade Marks (CGPDTM) .
Part 2: Instance when the compulsory license was granted and its aftereffects.
So far, only one compulsory license has been granted in India that meets the required criteria. In the case of Bayer Corporation v. Natco Pharma Limited, 2014(60) PTC 277 (BOM) , the landmark judgement of granting compulsory licensing for the pharmaceutical products was pronounced.
Facts:
- The case was filed by Bayer Corporation against the decision of the Intellectual Property Appellate Board (IPAB) to grant a compulsory license to Natco Pharmaceuticals for Nexavar (Sorafenib Tosylate), a drug to treat liver and kidney cancer .
- Bayer Corporation was selling the drug in India for Rs. 2,80,428, while Natco Pharmaceuticals requested a voluntary license for the drug with a price of Rs 8800, which is significantly lower than the price of the patent holder. The request was declined by Bayer Corporation .
- Only 2% of the total patients had access to medicine. Hence, Bayer Corporation was not able to fulfill the demand .
- After three years, Natco Pharmaceuticals applied for a compulsory license and got it at the rate of 7% royalty rate .
Hence, from the facts, it can be observed that,
Hence, all the conditions for obtaining a compulsory license under Section 84 of the Patents Act, 1970, were satisfied . A royalty rate of 7% was also fixed as per the norms given under Section 90 of the Patents Act, 1970 .
Judgement:
On the question of whether adequate efforts were made to obtain a compulsory license, the court found that Bayer’s response to NATCO dated December 27th, 2010, proves that voluntary license was applied by NATCO and the application was rejected by Bayer Corporation . While examining the case, the court also found out about the unavailability, unaffordability, and inaccessibility of the drug in the Indian Market . The petitioner could not satisfy the court regarding the working of invention in India as Bayer contended that mere imports would constitute to the working of the invention in India .
The court held that;
- The motive of patent granting is to utilize the invention for societal needs and further research and development . The abovementioned invention is unavailable, unaffordable, and inaccessible to the general public in India. Hence, the motive of granting a patent was not fulfilled. Hence, granting a compulsory license was justified.
- The Doha Agreement and the TRIPS agreement allow compulsory licensing in the hour of need, and the current scenario satisfied all the conditions . Hence, the compulsory license conformed with the international norms.
Talking about the aftereffects of this judgment, due to the lower price of the generic drug, compulsory licensing was seen as a medium to lower the price of expensive drugs, and applications for compulsory licenses for many other drugs were filed. However, in India, no compulsory license for pharmaceuticals was provided after this case. There can be two reasons behind that:
- Pressure and comments from Western countries.
- Due to Nexavar's compulsory license, the price of other drugs also came down.
Part 3: Comparison of patent rights and compulsory licensing in India.
Criteria Patent Rights Compulsory Licensing
Granting Authority Office of Controller General of Patents, Design, and Trademark Office of Controller General of Patents, Design, and Trademark
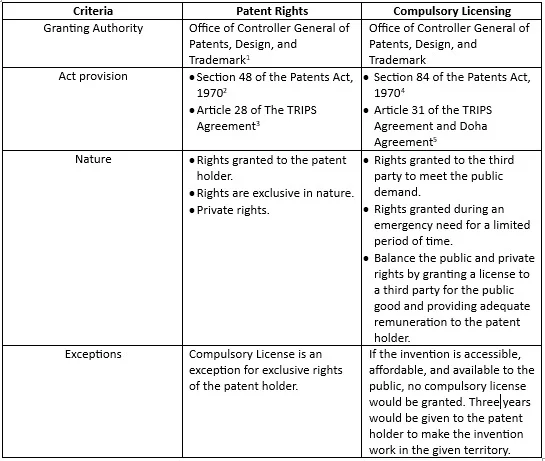
Exceptions Compulsory License is an exception for exclusive rights of the patent holder. If the invention is accessible, affordable, and available to the public, no compulsory license would be granted. A time of three years is given to the patent holder to make the invention work in the given territory.
Part 4: Author’s point of view and way ahead.
Compulsory licensing is a necessary provision to create deterrence for the public good and to manage situations in the hour of emergency. It maintains the balance between public and private rights and public and private good. It helps the consumers to get the drugs at an affordable price and overcome the shortage of drugs in the market. The case of Bayer Corporation v. Natco Pharma Limited, 2014(60) PTC 277 (BOM) established an example of the same.
However, this raises a concern over future innovations in biotechnology and pharmaceutical fields as it might discourage the innovators regarding their rights, or it might happen that they refuse to disclose the know-how of their inventions to the public. Hence, striking a balance between the patent rights and compulsory licensing is necessary to keep encouraging innovation and use it for the public good. For this, the following steps can be taken,
- Use of compulsory license as a deterrence only:
Using CL as deterrence will not demotivate the innovators. As witnessed after the case of Nexavar, the price of various drugs came down due to the fear of compulsory licensing. This way, compulsory license was never used further.
- Encouraging Voluntary Licensing:
Voluntary licensing is a way to license an invention by the patent holder on mutually agreed terms. It is a voluntary initiative by the patentee and not imposed on him.
Reference Notes:
GOODREADS, https://www.goodreads.com/quotes/10318150-the-right-to-swing-my-fist-ends-where-the-other (February 28, 2025).
Ved Prakash Patel, Compulsory License: The Exception to the Patent Rights, MANUPATRA ARTICLES (February 28, 2025), https://articles.manupatra.com/article-details/Compulsory-License-The-Exception-to-the-Patent-Rights.
The Patents Act, No. 39 of 1970, § 48, No. 39, Acts of Parliament, 1970 (India).
VAJIRAM & RAVI, http://vajiramandravi.com/quest-upsc-notes/compulsory-licensing/ (February 28, 2025).
The Patents Act, No. 39 of 1970, § 84, No. 39, Acts of Parliament, 1970 (India).
Agreement on Trade-Related Aspects of Intellectual Property Rights, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1869 U.N.T.S. 299.
Agreement on Trade-Related Aspects of Intellectual Property Rights art. 31, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1869 U.N.T.S. 299.
Bayer Corporation v. Natco Pharma Limited, 2014(60) PTC 277 (BOM).
The Patents Act, No. 39 of 1970, § 90, No. 39, Acts of Parliament, 1970 (India).
Ankit Rastogi, Bayer Corporation v. Union of India & Ors., INDIAN CASE LAW (April 08, 2020), https://indiancaselaw.in/bayer-corporation-ors-v-union-of-india-ors/.
Indian Controller General of Patents, Designs & Trademarks, CGPDTM, https://ipindia.gov.in.
Agreement on Trade-Related Aspects of Intellectual Property Rights art. 28, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1C, 1869 U.N.T.S. 299.
Bayer Corporation v. Natco Pharma Limited, 2014(60) PTC 277 (BOM).